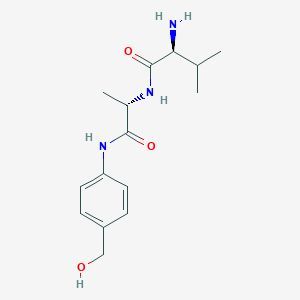
Val-Ala-PAB / 500 mg
Properties
| Signal Word | Warning |
Product Description
Val-Ala-PAB is a specialized chemical compound designed for specific applications within the scientific research community. It is known for its unique properties that make it suitable for various laboratory experiments and analytical procedures. This compound plays a crucial role in enhancing the efficiency and accuracy of certain reactions, making it an essential tool for researchers working in fields such as biochemistry, pharmaceuticals, and environmental science. Its precise formulation ensures stability under controlled conditions, allowing for consistent performance across different experimental setups.
Application
Val-Ala-PAB finds its primary application in biochemical assays where it acts as a substrate or reagent. Its ability to participate in specific enzymatic reactions makes it invaluable for enzyme activity measurements and kinetic studies. Additionally, it can be utilized in the development of new diagnostic tools and therapeutic agents, contributing to advancements in medical research and drug discovery processes.
Articles:
- Novel immunoconjugates comprised of streptonigrin and 17-amino-geldanamycin attached via a dipeptide-p-aminobenzyl-amine linker system
Publication Date: Available online 5 April 2009
Patrick J. Burke, Brian E. Toki, David W. Meyer, Jamie B. Miyamoto, Kim M. Kissler, Martha Anderson, Peter D. Senter, Scott C. Jeffrey
https://doi.org/10.1016/j.bmcl.2009.03.145
- Exploring alternative antibody scaffolds: Antibody fragments and antibody mimics for targeted drug delivery
Publication Date: Available online 8 November 2018
Daniel A. Richards
https://doi.org/10.1016/j.ddtec.2018.10.005
- Engineering Enzyme-Cleavable Oligonucleotides by Automated Solid-Phase Incorporation of Cathepsin B Sensitive Dipeptide Linkers
Publication Date: 25 December 2021
Dr. Cheng Jin, Dr. Afaf H. EI-Sagheer, Siqi Li, Prof. Katherine A. Vallis, Prof. Weihong Tan, Prof. Tom Brown