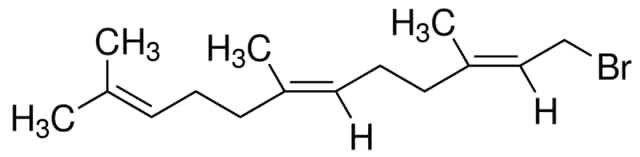
trans,trans-Farnesyl bromide / 1 G
Safety Information
Hazard Statements
Precautionary Statements
Pictograms
Properties
| Signal Word | Danger |
| Flash Point (C) | 113 °C - closed cup |
| Flash Point (F) | 235.4 °F - closed cup |
| Density | 1.052 g/mL at 25 °C (lit.) |
| Boiling Point | 100-110 °C/15 mmHg (lit.) |
Product Description
trans,trans-Farnesyl bromide is an organic compound featuring a brominated farnesyl group. It is used in organic synthesis and chemical research due to its reactivity and structural properties.
Application:
trans,trans-Farnesyl bromide is used as an intermediate in organic synthesis, particularly in the development of pharmaceuticals and other complex organic molecules.
Articles:
- Toxicity and Sublethal Effect of Farnesyl Acetate on Diamondback Moth, Plutella xylostella (L.) (Lepidoptera: Plutellidae)
Publication Date: 27 January 2021
Norazila Yusoff, Idris Abd Ghani, Nurul Wahida Othman, Wan Mohd Aizat and Maizom Hassan
https://doi.org/10.3390/insects12020109
- Synthesis and antimicrobial activity of geranyloxy- and farnesyloxy-acetophenone derivatives against oral pathogens
Publication Date: Available online 11 June 2012
Laetitia Bonifait, Annie Marquis, Salvatore Genovese, Francesco Epifano, Daniel Grenier
https://doi.org/10.1016/j.fitote.2012.06.003
- Synthesis and biological evaluation of farnesylthiosalicylamides as potential anti-tumor agents
Publication Date:
Yong Ling, Zhiqiang Wang, Hongyan Zhu, Xuemin Wang, Wei Zhang, Xinyang Wang, Li Chen, Zhangjian Huang, Yihua Zhang