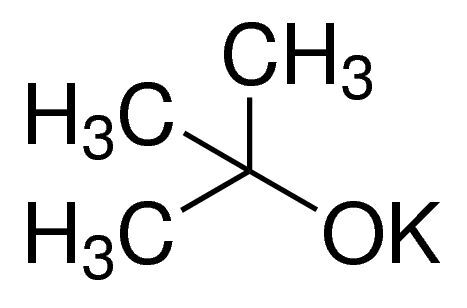
Potassium tert-butoxide solution / 50 ML
Safety Information
Hazard Statements
Precautionary Statements
Pictograms
Properties
| Signal Word | Danger |
| Flash Point (C) | -19 °C - closed cup |
| Flash Point (F) | -2.2 °F - closed cup |
| Density | 0.902 g/mL at 25 °C |
Product Description
POTASSIUM TERT-BUTOXIDE is a strong base commonly used in organic synthesis reactions. It is valued for its ability to deprotonate various compounds and initiate reactions such as deprotonation, condensation, and elimination.
Application:
POTASSIUM TERT-BUTOXIDE finds application as a base in organic synthesis, particularly in reactions requiring strong basic conditions. It is utilized in the synthesis of pharmaceuticals, agrochemicals, and specialty chemicals.
Articles:
- Potassium tert-butoxide in synthesis
Publication Date: February 1, 1974
D. E. Pearson and Calvin A. Buehler
https://doi.org/10.1021/cr60287a004
- The super-basic butyllithium/potassium tert-butoxide mixture and other LICKOR reagents
Publication Date: 1984
Schlosser, Manfred; Strunk, Sven
https://doi.org/10.1016/S0040-4039(01)80014-9
- Potassium tert-Butoxide-Catalyzed Dehydrogenative SiO Coupling: Reactivity Pattern and Mechanism of an Underappreciated Alcohol Protection
Publication Date: 24 February 2009
Andreas Weickgenannt, Martin Oestreich Prof. Dr