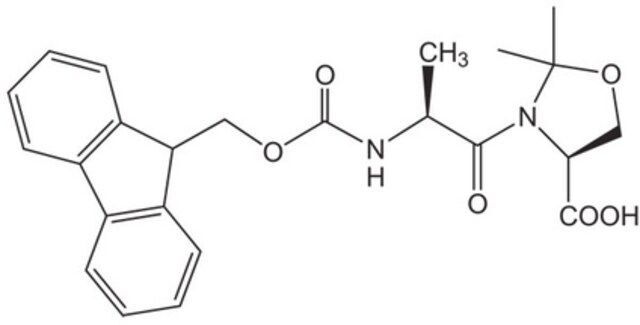
Fmoc-Ala-Ser(psiMe,Mepro)-OH / 25 G
Properties
| Flash Point (C) | Not available |
| Flash Point (F) | Not available |
Product Description
Description:
Fmoc-Ala-Ser(ψMe,Mepro)-OH is a high-purity pseudoproline dipeptide designed for use in solid-phase peptide synthesis (SPPS). This compound features an Fmoc (9-fluorenylmethoxycarbonyl) protecting group on the alanine residue and a ψ(Me,Mepro) pseudoproline modification on the serine residue. The pseudoproline moiety enhances peptide solubility and reduces aggregation during synthesis, making it an essential tool for constructing complex peptides with improved yield and purity. Manufactured under strict quality controls, this product is ideal for research and industrial applications requiring precise peptide assembly.
Applications:
- Peptide Synthesis: Widely used in SPPS to synthesize peptides with challenging sequences, particularly those prone to β-sheet formation or aggregation. The pseudoproline structure temporarily disrupts secondary structure formation, facilitating the coupling of subsequent amino acids.
- Pharmaceutical Research: Employed in the development of peptide-based therapeutics, such as enzyme inhibitors, receptor agonists/antagonists, and antimicrobial peptides.
- Biochemical Studies: Utilized in the preparation of peptide analogs for studying protein-protein interactions, enzyme-substrate specificity, and conformational properties.
- Custom Peptide Production: A key building block for contract research organizations (CROs) and peptide manufacturers producing tailored sequences for academic and industrial clients.
Usage:
Fmoc-Ala-Ser(ψMe,Mepro)-OH is typically incorporated into peptide chains using standard Fmoc-based SPPS protocols. It is compatible with automated peptide synthesizers and manual synthesis workflows. The pseudoproline group is cleaved during the final deprotection step (e.g., with TFA), reverting the serine residue to its native form. Recommended coupling reagents include HBTU, HATU, or DIC/HOBt, with DIEA or NMM as bases, depending on the synthesis conditions.
Handling and Storage:
- Store at -20°C in a dry, dark environment to maintain stability.
- Avoid prolonged exposure to moisture or acidic conditions prior to use.
- Handle under inert gas (e.g., argon or nitrogen) for long-term storage to prevent degradation.
Packaging: Available in 1 g, 5 g, and bulk quantities upon request. Supplied with a Certificate of Analysis (CoA) confirming purity and identity.
Advantages:
- Enhances solubility and coupling efficiency in peptide synthesis.
- Reduces synthesis failures caused by aggregation or poor solubility.
- High compatibility with standard Fmoc chemistry protocols.