Cadmium Hexacyanoferrate / 100 g
Product Description
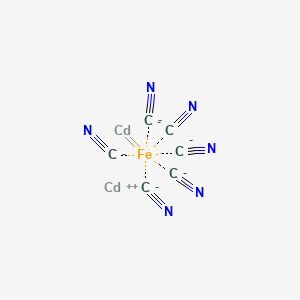
Cadmium hexacyanoferrate is a coordination compound consisting of cadmium ions bound to a hexacyanoferrate(II) ion. It is known for its distinctive properties, including its ability to act as a catalyst in certain chemical reactions. This compound is particularly interesting due to its potential applications in electrochemistry and energy storage technologies. Cadmium hexacyanoferrate exhibits redox activity, making it useful in systems that require the conversion of electrical energy into chemical energy and vice versa. Its stability and reversibility in these processes make it a promising candidate for developing advanced batteries and supercapacitors.
Application
In practical applications, cadmium hexacyanoferrate is explored for its role in energy storage devices, such as rechargeable batteries and supercapacitors. Its ability to facilitate electron transfer reactions makes it valuable in the construction of electrodes that can store and release energy efficiently. Additionally, its use in electrochemical sensors and actuators is being investigated, leveraging its redox properties to detect specific substances or perform mechanical actions in response to chemical changes.
Articles:
- The formation of bilayered nickel-iron, cadmium-iron and cadmium—silver hexacyanoferrates by an electrochemically driven insertion—substitution mechanism
Publication Date: Available online 21 February 1999
Aleš Dostal, Michael Hermes, Fritz Scholz
https://doi.org/10.1016/S0022-0728(96)04709-2
- On the electrochemically driven formation of bilayered systems of solid Prussian-blue-type metal hexacyanoferrates: a model for Prussian blue ∣ cadmium hexacyanoferrate supported by finite difference simulations
Publication Date: Available online 9 April 2001
Michael Hermes, Milivoj Lovrić, Monika Hartl, Utz Retter, Fritz Scholz
https://doi.org/10.1016/S0022-0728(00)00527-1
- Investigation of the structure-reactivity relationship in the platinum/metal cadmium hexacyanoferrate (Pt/MxCdFe(CN)6)-modified electrode system
Publication Date: February 1, 1992
Carmela H. Luangdilok, Douglas J. Arent, Andrew B. Bocarsly, and Robert Wood