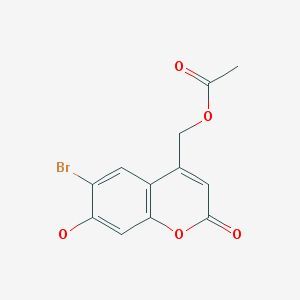
6-Bromo-7-hydroxycoumarin-4-ylmethyl acetate / 100 g
Product Description
6-Bromo-7-hydroxycoumarin-4-ylmethyl acetate is a derivative of coumarin, a heterocyclic compound known for its wide range of biological activities. This particular derivative incorporates bromine and hydroxy groups, which can influence its chemical reactivity and biological properties. Coumarins and their derivatives have been extensively studied for their anticoagulant, antimicrobial, and anticancer activities, among others. The introduction of the acetate ester further modifies the compound's physicochemical properties, potentially enhancing its bioavailability or solubility in certain applications.
Application
In practical applications, 6-Bromo-7-hydroxycoumarin-4-ylmethyl acetate could be explored for its potential in pharmaceuticals, particularly in the development of drugs targeting blood coagulation disorders or cancer. Its modified coumarin structure may offer advantages in drug design, such as increased potency or specificity. Additionally, the compound's properties suggest it could be useful in research related to blood clotting mechanisms or as a scaffold for the synthesis of more complex bioactive molecules.
Articles:
- 6-Bromo-7-hydroxy-3-methylcoumarin (mBhc) is an efficient multi-photon labile protecting group for thiol caging and three-dimensional chemical patterning
Publication Date: 03 Aug 2016
M. Mohsen Mahmoodi, Stephanie A. Fisher, Roger Y. Tam, Philip C. Goff, Reid B. Anderson, Jane E. Wissinger, David A. Blank, Molly S. Shoichetb and Mark D. Distefano
https://doi.org/10.1039/C6OB01045H
- Photoclick chemistry: a fluorogenic light-triggered in vivo ligation reaction
Publication Date: Available online 11 July 2014
Carlo P Ramil, Qing Lin
https://doi.org/10.1016/j.cbpa.2014.05.024
- New Caged Coumarin Fluorophores with Extraordinary Uncaging Cross Sections Suitable for Biological Imaging Applications
Publication Date: March 20, 2004
YuRui Zhao, Quan Zheng, Kenneth Dakin, Ke Xu, Manuel L. Martinez, and Wen-Hong Li