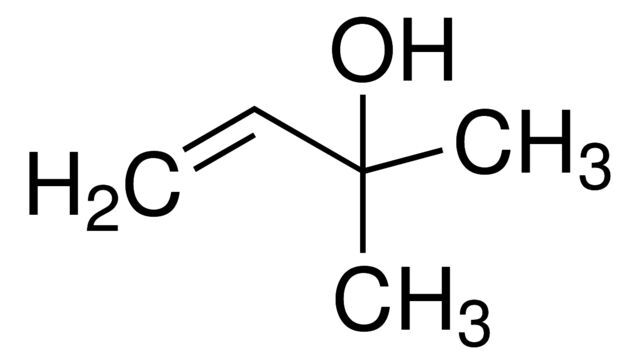
2-Methyl-3-buten-2-ol / 1 L
Safety Information
Hazard Statements
Precautionary Statements
Pictograms
Properties
| Signal Word | Danger |
| Flash Point (C) | 11 °C - closed cup |
| Flash Point (F) | 51.8 °F - closed cup |
| Vapor Pressure | 51 mmHg ( 25 °C) |
| Density | 0.824 g/mL at 25 °C (lit.) |
| Boiling Point | 98-99 °C (lit.) |
Product Description
2-Methyl-3-buten-2-ol is a chemical compound commonly used as a building block in the synthesis of flavors, fragrances, and pharmaceuticals. It is characterized by its unsaturated structure and alcohol functional group.
Application:
2-Methyl-3-buten-2-ol is utilized as a key intermediate in the production of flavoring agents, such as raspberry ketone, and fragrances, like lilac and jasmine. It also serves as a precursor in the synthesis of pharmaceutical compounds, contributing to various medicinal applications.
Articles:
- 2-methyl-3-buten-2-ol: A Pheromone Component of Conifer Bark Beetles Found in the Bark of Nonhost Deciduous Trees
Publication Date: 10 Apr 2012
Qing-He Zhang, Fredrik Schlyter and Göran Birgersson
https://doi.org/10.1155/2012/414508
- Comprehensive study of the thermodynamic properties for 2-methyl-3-buten-2-ol
Publication Date: Available online 29 July 2015
Dzmitry Zaitsau, Eugene Paulechka, Dzmitry S. Firaha, Andrey V. Blokhin, Gennady J. Kabo, Ala Bazyleva, Andrey G. Kabo, Mikhail A. Varfolomeev, Viktor M. Sevruk
https://doi.org/10.1016/j.jct.2015.07.028
- Hydroxyaldehyde Products from Hydroxyl Radical Reactions of Z-3-Hexen-1-ol and 2-Methyl-3-buten-2-ol Quantified by SPME and API-MS
Publication Date: September 10, 2003
Fabienne Reisen, Sara M. Aschmann, Roger Atkinson, and Janet Arey