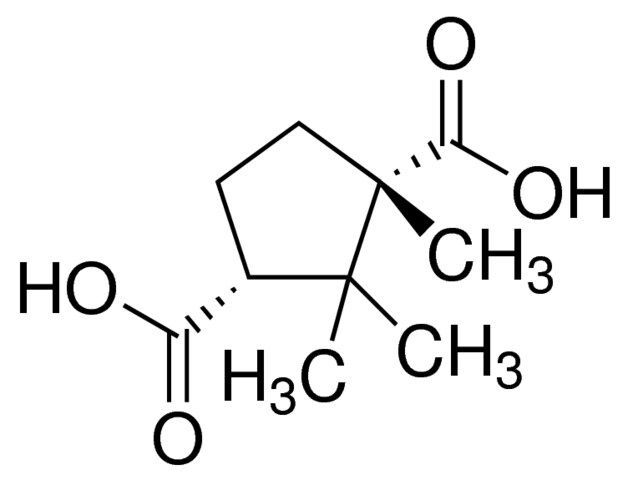
(1S,3R)-(−)-Camphoric acid / 1 G
Properties
| Flash Point (C) | Not available |
| Flash Point (F) | Not available |
| Melting Point | 188-190 °C (lit.) |
Product Description
(1S,3R)-(−)-Camphoric acid is a chiral compound with a specific stereochemical arrangement. It is commonly employed in organic synthesis, serving as a key intermediate for the production of pharmaceuticals, agrochemicals, and fine chemicals.
Application:
Utilized in organic chemistry, (1S,3R)-(−)-Camphoric acid acts as a crucial building block for the synthesis of various chemical compounds. Its application is particularly significant in the manufacturing of pharmaceuticals, agrochemicals, and fine chemical products.
Articles:
- 1 D Supramolecular Double-Chains based on π-π Stacking Interactions: Synthesis and Crystal Structure of [Cu(phen)(C10H16O4)] · 3 H2O with phen = 1,10-phenanthroline
Publication Date: 16 May 2000
Yue-Qing Zheng Prof. Dr., Jie Sun, Jian-Li Lin
https://doi.org/10.1002/(SICI)1521-3749(200006)626:6<1274::AID-ZAAC1274>3.0.CO;2-J
- Crystal structure and phase transition of bis-aqua-sebacato magnesium Mg(C10H16O4)2(H2O)2
Publication Date: Available online 18 May 2011
Adel Mesbah, Lionel Aranda, Jean Steinmetz, Emmanuel Rocca, Michel François
https://doi.org/10.1016/j.solidstatesciences.2011.05.008
- (1R,3S)-Camphoric acid as a building block in supramolecular chemistry: adducts with organic polyamines
Publication Date: 2003
C. M. Zakaria, G. Ferguson, A. J. Lough and C. Glidewell