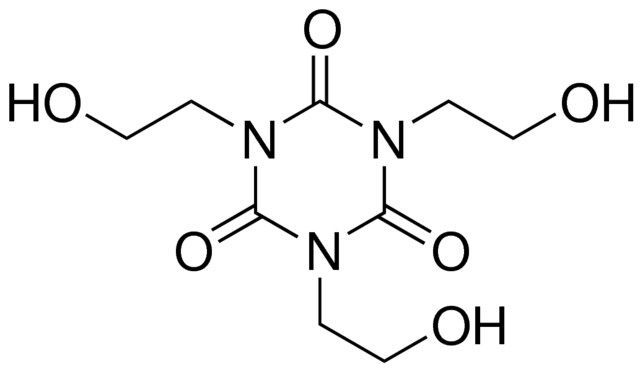
1,3,5-Tris(2-hydroxyethyl)isocyanurate / 50 G
Properties
| Flash Point (C) | 241 °C - open cup |
| Flash Point (F) | 465.8 °F - open cup |
| Melting Point | 136-140 °C (lit.) |
Product Description
1,3,5-Tris(2-hydroxyethyl)isocyanurate is a chemical compound commonly used as a crosslinking agent and flame retardant in the production of polyurethane foams and coatings. It contains three hydroxyethyl groups attached to an isocyanurate ring structure.
Application:
1,3,5-Tris(2-hydroxyethyl)isocyanurate serves as a crosslinking agent in the synthesis of polyurethane foams, coatings, and adhesives, enhancing their strength and durability. It is also employed as a flame retardant additive, improving the fire resistance of various polymeric materials.
Articles:
- New Hydrogen-Bond-Enriched 1,3,5-Tris(2-hydroxyethyl) Isocyanurate Covalently Functionalized MCM-41: An Efficient and Recoverable Hybrid Catalyst for Convenient Synthesis of Acridinedione Derivatives
Publication Date: November 12, 2019
Zahra Alirezvani, Mohammad G. Dekamin and Ehsan Valiey
https://doi.org/10.1021/acsomega.9b02755
- Dendrons containing boric acid and 1,3,5-tris(2-hydroxyethyl)isocyanurate covalently attached to silica-coated magnetite for the expeditious synthesis of Hantzsch esters
Publication Date: 27 January 2021
Mahsa Sam, Mohammad G. Dekamin & Zahra Alirezvani
https://doi.org/10.1038/s41598-020-80884-z
- 1,3,5-Tris(2-hydroxyethyl)isocyanurate functionalized graphene oxide: a novel and efficient nanocatalyst for the one-pot synthesis of 3,4-dihydropyrimidin-2(1H)-ones
Publication Date: 13 Jun 2017
Mohammad G. Dekamin, Fatemeh Mehdipoora and Amene Yaghoubia