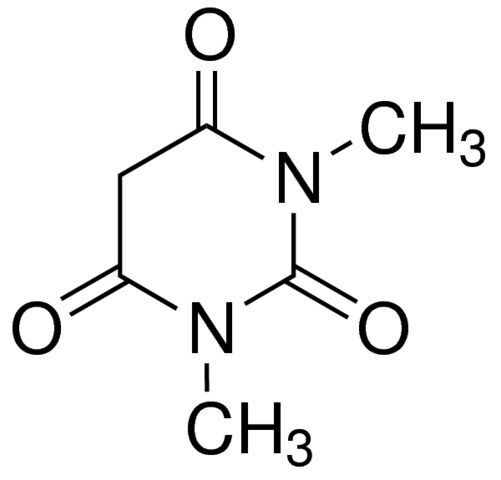
1,3-Dimethylbarbituric acid / 250 G
Safety Information
Hazard Statements
Precautionary Statements
Pictograms
Properties
| Signal Word | Danger |
| Flash Point (C) | Not available |
| Flash Point (F) | Not available |
| Melting Point | 121-123 °C (lit.);123-126 °C; |
Product Description
1,3-DIMETHYLBARBITURIC ACID is a chemical compound commonly utilized as a precursor in the synthesis of pharmaceuticals and herbicides. It is characterized by its barbiturate structure and is known for its role in organic synthesis.
Application:
1,3-DIMETHYLBARBITURIC ACID finds application as a key intermediate in the production of barbiturate-based pharmaceuticals and herbicides. It is utilized in organic synthesis processes to create compounds with sedative and herbicidal properties.
Articles:
- Metal-ligand cooperative iridium complex catalyzed C-alkylation of oxindole and 1,3-dimethylbarbituric acid using alcohols
Publication Date: Available online 16 July 2022
Ao Song, Yunbo Liu, Xuan Jin, Dianrun Su, Zhaopeng Li, Shengsheng Yu, Lingbao Xing, Xiangchao Xu, Rongzhou Wang, Feng Li
https://doi.org/10.1016/j.gresc.2022.07.002
- Reactions of 5-dihydrocotarnyl-1,3-dimethylbarbituric acid and other cotarnine derivatives with 1,3-dimethylbarbituric acid. X-ray diffraction analysis of a 5,5-spiro derivative of 1,3-dimethylbarbituric acid
Publication Date: August 2002
K. A. Krasnov, V. G. Kartsev & V. N. Khrustalev
https://doi.org/10.1023/A:1020983527851
- Synthesis, Structure and Reactions of 1,3-Dimethyl-5-bis(thiomethyl)methylenebarbituric Acid
Publication Date: June 2, 2014
Kamal Sweidan EMAIL logo , Ahmed Abu-Rayyan , Ahmad Al-Sheikh , Cäcilia Maichle-Mößmer , Manfred Steimann and Norbert Kuhn