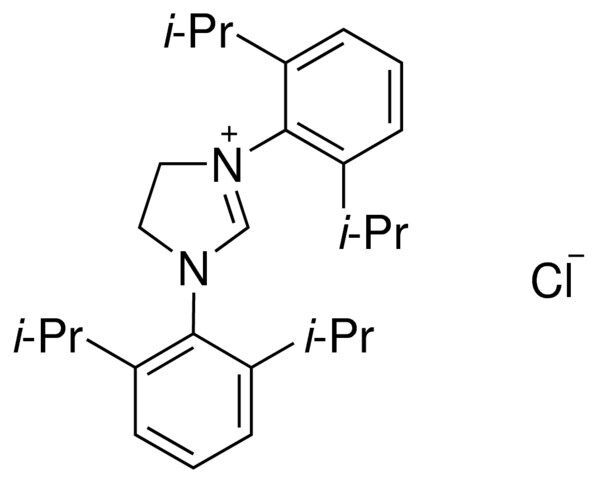
1,3-Bis-(2,6-diisopropylphenyl)imidazolinium chloride / 100 KG
Safety Information
Hazard Statements
Precautionary Statements
Pictograms
Properties
| Signal Word | Warning |
Product Description
1,3-Bis-(2,6-diisopropylphenyl)imidazolinium chloride is a potent organocatalyst designed for use in a variety of chemical reactions. It is recognized for its exceptional stability and strong acidity, which enable it to effectively catalyze reactions that are challenging to perform with traditional methods. This compound's unique structure allows it to interact favorably with a wide range of substrates, leading to enhanced reaction rates and improved product selectivity. Its utility is not limited to academic research; it also finds application in industrial settings, particularly in the production of fine chemicals and pharmaceuticals, where precise control over reaction outcomes is crucial.
Application
In the realm of chemistry, 1,3-Bis-(2,6-diisopropylphenyl)imidazolinium chloride serves as an organocatalyst, facilitating reactions that benefit from its acidic nature and structural features. Its use spans from laboratory-scale experiments to industrial processes, playing a significant role in the synthesis of pharmaceuticals, agrochemicals, and other specialty chemicals. This compound's ability to enhance reaction efficiency and product quality positions it as a valuable asset in the pursuit of sustainable and environmentally friendly chemical manufacturing processes.
Articles:
- Efficient synthetic protocols for the preparation of common N-heterocyclic carbene precursors
Publication Date: 25 Nov 2015
Morgan Hans, Jan Lorkowski, Albert Demonceau and Lionel Delaude
https://doi.org/10.3762/bjoc.11.252
- Synthesis of bis-oxazoline-ruthenium(II)-arene complexes.: Combined catalytic isomerisation and Claisen rearrangement of bis-allyl ether
Publication Date: Available online 12 October 2002
Hamed Ben Ammar, Jérôme Le Nôtre, Mansour Salem, Mohamed T Kaddachi, Pierre H Dixneuf
https://doi.org/10.1016/S0022-328X(02)01881-8
- A Comparative Study on (NHC)Pd(acac)Cl Complexes (NHC = N-heterocyclic carbene): Indications for the Origin of the Different Reactivity of Saturated and Unsaturated NHC in Cross-Coupling Reactions
Publication Date: September 9, 2009
Ole H. Winkelmann, Andrei Riekstins, Steven P. Nolan and Oscar Navarro