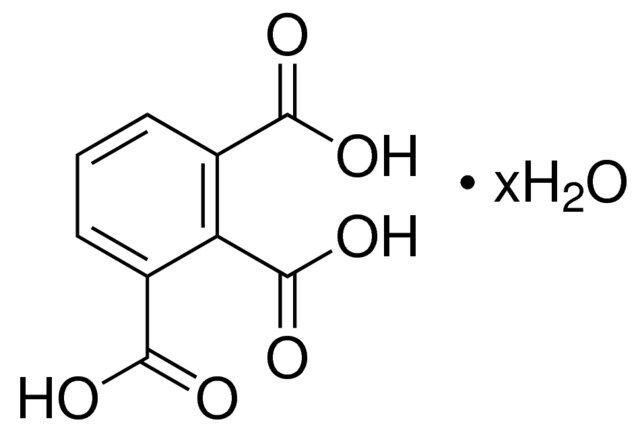
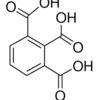
1,2,3-Benzenetricarboxylic acid hydrate / 5 G
Safety Information
Hazard Statements
Precautionary Statements
Pictograms
Properties
| Signal Word | Warning |
| Flash Point (C) | Not available |
| Flash Point (F) | Not available |
| Melting Point | ~195 °C (dec.) |
Product Description
1,2,3-Benzentricarboxylic acid hydrate is a crystalline compound with three carboxylic acid groups. It is commonly used as a monomer in the synthesis of polymeric materials, particularly in the production of high-performance engineering plastics.
Application:
1,2,3-Benzentricarboxylic acid hydrate serves as a monomer in the synthesis of polymeric materials, especially in the production of engineering plastics like polyesters and polyamides. These materials find application in various industries including automotive, electronics, and packaging.
Articles:
- Suppression of PFKFB3-driven glycolysis restrains endothelial-to-mesenchymal transition and fibrotic response
Publication Date: 01 September 2022
Hao Zeng, Ting Pan, Meiling Zhan, Renaguli Hailiwu, Baolin Liu, Hua Yang & Ping Li
https://doi.org/10.1038/s41392-022-01097-6
- Spectroscopic and Structural Study of a New Conducting Pyrazolium Salt
Publication Date: 31 July 2021
Sylwia Zięba, Agata Piotrowska, Adam Mizera, Paweł Ławniczak, Karolina H. Markiewicz, Andrzej Gzella, Alina T. Dubis and Andrzej Łapiński
https://www.mdpi.com/1420-3049/26/15/4657#
- Single-Molecule-Based Electroluminescent Device as Future White Light Source
Publication Date: January 3, 2019
Muhammad Usman, Krishna Prasad Bera, Golam Haider, Batjargal Sainbileg, Michitoshi Hayashi, Gene-Hsiang Lee, Shie-Ming Peng, Yang-Fang Chen and Kuang-Lieh Lu