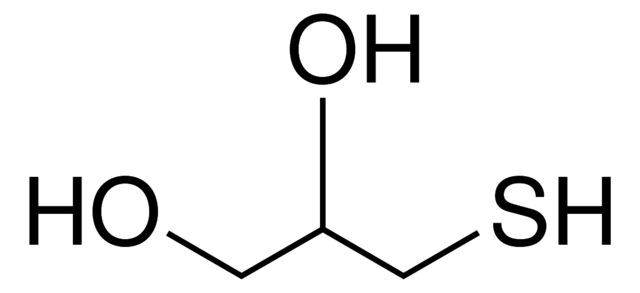
1-Thioglycerol / 2.5 L
Safety Information
Hazard Statements
Precautionary Statements
Pictograms
Properties
| Signal Word | Danger |
| Flash Point (C) | 99 °C - closed cup |
| Flash Point (F) | 210.2 °F - closed cup |
| Density | 1.25 g/mL at 25 °C (lit.) |
| Boiling Point | 118 °C/5 mmHg (lit.) |
Product Description
1-Thioglycerol is a versatile chemical compound with a mercaptan functional group, offering unique reactivity in various biochemical applications. Recognized for its distinct properties, this compound serves as an essential reagent in diverse laboratory settings.
Application:
Commonly utilized as a reducing agent and protein denaturant, 1-Thioglycerol finds application in molecular biology experiments, particularly in RNA research and protein purification processes. Its ability to break disulfide bonds makes it a valuable tool for maintaining the stability of biological samples during experimentation.
Articles:
- Contribution of the Loss of Nanocrystal Ligands to Interdot Coupling in Films of Small CdSe/1-Thioglycerol Nanocrystal
Publication Date:June 5, 2003
Dae I. Kim, Mohammad A. Islam, Luis Avila, and Irving P. Herman
https://doi.org/10.1021/jp030168h
-
-Photocurrent mechanism in a hybrid system of 1-thioglycerol-capped HgTe nanoparticles
Publication Date: October 06 2003
Hyunsuk Kim; Kyoungah Cho; Hyunwoo Song; Byungdon Min; Jong-Soo Lee; Gyu-Tae Kim; Sangsig Kim; Sung Hyun Kim; Taeyong Noh
https://doi.org/10.1063/1.1631052
- -The effect of surface attachment on ligand binding: studying the association of Mg2+, Ca2+ and Sr2+ by 1-thioglycerol and 1,4-dithiothreitol monolayers
Publication Date: November 01 2005
Doron Burshtaina and Daniel Mandler