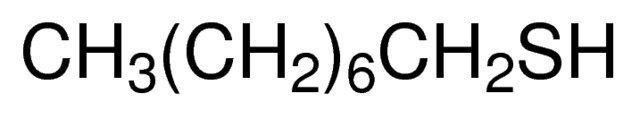1-Octanethiol / 5 L
Safety Information
Hazard Statements
Precautionary Statements
Pictograms
Properties
| Signal Word | Warning |
| Flash Point (C) | 70 °C |
| Flash Point (F) | 158.0 °F |
| Density | 0.843 g/mL at 25 °C (lit.) |
| Boiling Point | 197-200 °C (lit.) |
Product Description
1-Octanethiol is a chemical compound consisting of a linear eight-carbon alkane chain with a thiol group (-SH) at one end. It is a colorless liquid with a strong, unpleasant odor resembling rotten eggs.
Application:
This compound is commonly used as a reagent in organic synthesis, particularly in the production of sulfur-containing compounds and as a precursor for pharmaceuticals, pesticides, and fragrances. It also finds application as a chemical intermediate and in industries such as coatings, adhesives, and lubricants.
Articles:
- Dispersion stability of 1-octanethiol coated Cu nanoparticles in a 1-octanol solvent for the application of nanoink
Publication Date: Available online 13 May 2014
Danee Cho, Jong-Hwan Baik, Da-hyun Choi, Caroline Sunyong Lee
https://doi.org/10.1016/j.apsusc.2014.04.203
- Oxidation Prevention Properties of Reduced Graphene Oxide Mixed with 1-Octanethiol-Coated Copper Nanopowder Composites
Publication Date: 13 Apr 2016
Danee Cho, Dahyun Choi, Youngjun Pyo, Rajendra C. Pawar, Yongil Kim, Eric H. Yoon and Caroline Sunyong Lee
https://doi.org/10.1155/2016/1050183
- Effect of 1-octanethiol as an electrolyte additive on the performance of the iron-air battery electrodes
Publication Date: 29 June 2020
R. D. McKerracher, H. A. Figueredo-Rodriguez, K. Dimogiannis, C. Alegre, N. I. Villanueva-Martinez, M. J. Lázaro, V. Baglio, A. S. Aricò & C. Ponce de Leόn