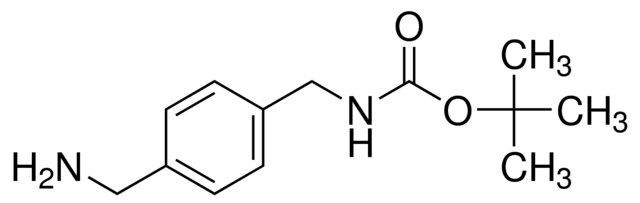
1-(N-Boc-aminomethyl)-4-(aminomethyl)benzene / 1 G
Safety Information
Hazard Statements
Precautionary Statements
Pictograms
Properties
| Signal Word | Warning |
| Flash Point (C) | Not available |
| Flash Point (F) | Not available |
| Melting Point | 64-68 °C (lit.) |
Product Description
1-(N-BOC-aminomethyl)-4-(aminomethyl)benzene is a chemical compound frequently employed as a building block in organic synthesis. With a BOC (tert-butyloxycarbonyl) protecting group, it facilitates controlled reactions in the creation of various organic compounds.
Application:
Utilized in organic chemistry, 1-(N-BOC-aminomethyl)-4-(aminomethyl)benzene serves as a crucial building block for the synthesis of diverse organic compounds, particularly in pharmaceutical and specialty chemical manufacturing.
Articles:
- Characterization of the Reduction of Oxygen at Anthraquinone-Modified Glassy Carbon and Highly Oriented Pyrolytic Graphite Electrodes
Publication Date: 11 Jul 2017
Izzet Koçak
https://doi.org/10.1080/00032719.2016.1236126
- A novel boronic acid-based fluorescent sensor for selectively recognizing Fe3+ ion in real time
Publication Date: 2019
Guiqian Fang, Hao Wang, Zhancun Bian, Min Guo, Zhongyu Wu and Qingqiang Yao
https://doi.org/10.1039/C9RA03978C
- Parallel Synthesis of an Imidazole-4,5-dicarboxamide Library Bearing Amino Acid Esters and Alkanamines
Publication Date: 15 December 2008
Rosanna Solinas, John C. DiCesare and Paul W. Baures