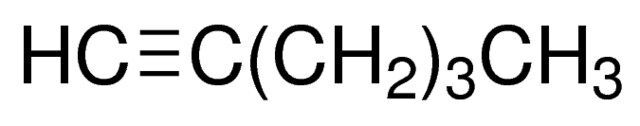1-Hexyne / 100 ML
Safety Information
Hazard Statements
Precautionary Statements
Pictograms
Properties
| Signal Word | Danger |
| Flash Point (C) | -20 °C - closed cup |
| Flash Point (F) | -4.0 °F - closed cup |
| Vapor Pressure | 253 mmHg ( 37.7 °C) |
| Density | 0.715 g/mL at 25 °C (lit.) |
| Boiling Point | 71-72 °C (lit.) |
| Melting Point | −132 °C (lit.) |
Product Description
1-Hexyne is an alkyne hydrocarbon with a triple bond between the first and second carbon atoms in its six-carbon chain. It is commonly used as a building block in organic synthesis.
Application:
1-Hexyne is utilized in organic synthesis for the preparation of various pharmaceuticals, agrochemicals, and specialty chemicals due to its reactive alkyne group.
Articles:
- Transhydrogenation of pentane and 1-hexyne over CrOx/Al2O3 and potassium-doped CrOx/Al2O3 catalysts
Publication Date: 19 June 2019
Mustapha D. Garba & S. David Jackson
https://doi.org/10.1007/s13203-019-0231-3
- Evidence of metallocycle formation by decomposition of 1-hexyne on Ru(0001): a RAIRS study
Publication Date: Available online 6 January 2002
Ana R Garcia, Ricardo Brito de Barros, Laura M Ilharco
https://doi.org/10.1016/S0039-6028(01)01929-X
- Mesostructured silicas as supports for palladium-catalyzed hydrogenation of phenyl acetylene and 1-phenyl-1-hexyne to alkenes
Publication Date: Available online 28 December 2005
Norman Marín-Astorga, Gina Pecchi, Thomas J. Pinnavaia, Gabriela Alvez-Manoli, Patricio Reyes