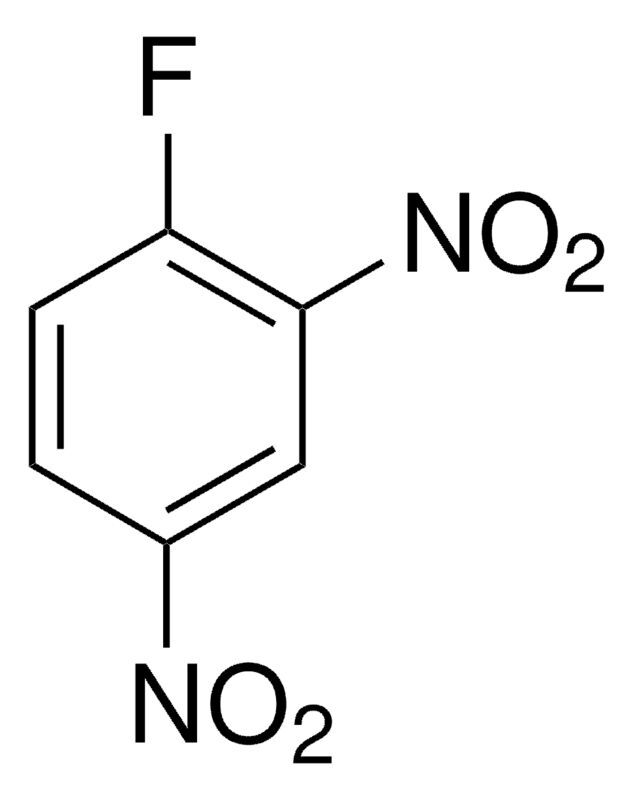
1-Fluoro-2,4-dinitrobenzene / 10 X 1 G
Safety Information
Hazard Statements
Precautionary Statements
Pictograms
Properties
| Signal Word | Warning |
| Flash Point (C) | 164 °C |
| Flash Point (F) | 327.2 °F |
| Boiling Point | 178 °C/25 mmHg (lit.) |
| Melting Point | 25-27 °C (lit.) |
Product Description
1-Fluoro-2,4-dinitrobenzene is a chemical compound utilized as a building block in the synthesis of pharmaceuticals, agrochemicals, and specialty chemicals. It is valued for its reactivity and ability to introduce fluorine-containing functional groups into organic molecules.
Application:
1-Fluoro-2,4-dinitrobenzene is commonly employed as a fluorinating agent in organic synthesis, enabling the introduction of fluorine atoms into various compounds. It is utilized in the pharmaceutical and agrochemical industries for the production of fluorinated intermediates and final products.
Articles:
- Reaction of Myosin with 1-Fluoro-2,4-dinitrobenzene at Low Ionic Strength
Publication Date: Available online 10 February 1969
M Bárány, G Bailin, K Bárány
https://doi.org/10.1016/S0021-9258(18)94404-2
- The α-Effect in SNAr Reaction of 1-Fluoro-2,4-dinitrobenzene with Hydrazine: Ground-State Destabilization versus Transition-State Stabilization
Publication Date: 2014.08.20
Cho, Hyo-Jin; Um, Ik-Hwan
https://doi.org/10.5012/bkcs.2014.35.8.2371
- Molecular Rearrangement in the Reaction of Cysteine with 1-Fluoro-2,4-Dinitrobenzene
Publication Date: 04 January 1958
H. P. BURCHFIELD