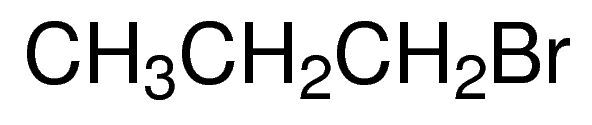1-Bromopropane / 5 ML
Safety Information
Hazard Statements
Precautionary Statements
Pictograms
Properties
| Signal Word | Danger |
| Flash Point (C) | 22 °C - closed cup |
| Flash Point (F) | 71.6 °F - closed cup |
| Vapor Density | 4.3 (vs air) |
| Vapor Pressure | 146 mmHg ( 20 °C) |
| Density | 1.354 g/mL at 25 °C (lit.) |
| Boiling Point | 71 °C (lit.) |
| Melting Point | −110 °C (lit.) |
Product Description
1-Bromopropane is a chemical compound commonly used as a solvent and intermediate in organic synthesis. It is a halogenated hydrocarbon with a wide range of industrial applications.
Application:
1-Bromopropane finds application as a solvent in various industrial processes, such as degreasing, cleaning, and as a reaction medium in organic synthesis. It is also utilized as a starting material in the production of pharmaceuticals and agrochemicals.
Articles:
- Neurotoxicity of 1-bromopropane: Evidence from animal experiments and human studies
Publication Date: Available online 17 May 2011
Gaku Ichihara, Junzoh Kitoh, Weihua Li, Xuncheng Ding, Sahoko Ichihara, Yasuhiro Takeuchi
https://doi.org/10.1016/j.jare.2011.04.005
- Biochemical Changes in the Central Nervous System of Rats Exposed to 1-Bromopropane for Seven Days
Publication Date: 01 May 2002
Hailan Wang, Gaku Ichihara, Hidenori Ito, Kanefusa Kato, Junzoh Kitoh, Tetsuya Yamada, Xiaozhong Yu, Seiji Tsuboi, Yoshinori Moriyama, Rie Sakatani, Eiji Shibata, Michihiro Kamijima, Seiichiro Itohara, Yasuhiro Takeuchi
https://doi.org/10.1093/toxsci/67.1.114
- The atmospheric degradation of 1-bromopropane (CH3CH2CH2Br): The photochemistry of bromoacetone
Publication Date: 04 September 2002
James B. Burkholder, Mary K. Gilles, Tomasz Gierczak, A. R. Ravishankara