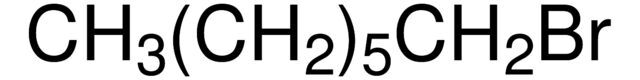1-Bromoheptane / 500 G
Safety Information
Hazard Statements
Precautionary Statements
Pictograms
Properties
| Signal Word | Warning |
| Flash Point (C) | 60 °C - closed cup |
| Flash Point (F) | 140.0 °F - closed cup |
| Vapor Density | 6.18 (vs air) |
| Density | 1.14 g/mL at 25 °C (lit.) |
| Boiling Point | 180 °C (lit.) |
| Melting Point | −58 °C (lit.) |
Product Description
1-Bromoheptane is an organic halide with a bromine atom attached to the first carbon of a heptane chain. It is commonly used in organic synthesis.
Application:
1-Bromoheptane is used as an alkylating agent in organic synthesis, facilitating the introduction of heptyl groups into various chemical compounds.
Articles:
- Application of a low power/reduced pressure helium ICP ionization source for mass spectrometric detection of organobromine compounds and derivatized organotin compounds
Publication Date: 7th January 2000
Joseph W. Waggoner, Lisa S. Milstein, Mikhail Belkin, Karen L. Sutton, Joseph A. Caruso and Harry B. Fannin
https://doi.org/10.1039/A905906G
- Protective effect of NAC against malathion-induced oxidative stress in freshly isolated rat hepatocytes
Publication Date: 2012 Mar 15
Sara Mostafalou, Mohammad Abdollahi, Mohammad Ali Eghbal and Nazli Saeedi Kouzehkonani
https://doi.org/10.5681%2Fapb.2012.011
- Synthesis and evaluation of quinonoid compounds against tumor cell lines
Publication Date: Available online 9 November 2010
Eufrânio N. da Silva Jr., Bruno C. Cavalcanti, Tiago T. Guimarães, Maria do Carmo F.R. Pinto, Igor O. Cabral, Cláudia Pessoa, Letícia V. Costa-Lotufo, Manoel O. de Moraes, Carlos K.Z. de Andrade, Marcelo R. dos Santos, Carlos A. de Simone, Marilia O.F. Goulart, Antonio V. Pinto