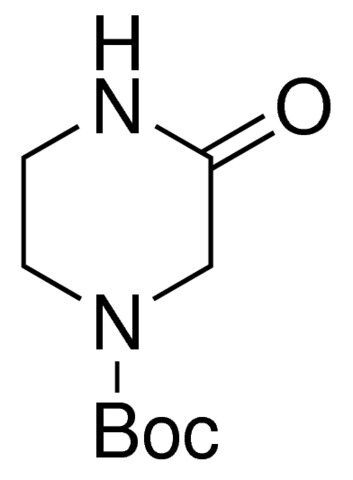
1-Boc-3-oxopiperazine / 1 G
Safety Information
Hazard Statements
Precautionary Statements
Pictograms
Properties
| Signal Word | Warning |
| Flash Point (C) | Not available |
| Flash Point (F) | Not available |
| Melting Point | 156-160 °C (lit.) |
Product Description
1-BOC-3-oxopiperazine is a chemical compound commonly used as a protecting group in organic synthesis. It features a BOC (tert-butyloxycarbonyl) group, providing stability to specific functional groups during various chemical reactions.
Application:
Widely employed in organic chemistry, 1-BOC-3-oxopiperazine serves as a key reagent for protecting amino groups, enabling controlled and selective reactions in the synthesis of pharmaceuticals and specialty chemicals.
Articles:
- Design, synthesis, and mechanistic study of 2-piperazineone-bearing peptidomimetics as novel HIV capsid modulators
Publication Date: 02 Jun 2023
Xujie Zhang, Lin Sun, Shujing Xu, Tianguang Huang, Fabao Zhao, Dang Ding, Chuanfeng Liu, Xiangyi Jiang, Yucen Tao, Dongwei Kang, Erik De Clercq, Christophe Pannecouque, Simon Cocklin, Alexej Dick, Xinyong Liu and Peng Zhan
https://doi.org/10.1039/D3MD00134B
- Identification of a potent and selective phosphatidylinositol 3-kinase δ inhibitor for the treatment of non-Hodgkin's lymphoma
Publication Date: Available online 8 October 2020
Wei-Qiong Zuo, Rong Hu, Wan-Li Wang, Yong-Xia Zhu, Ying Xu, Luo-Ting Yu, Zhi-Hao Liu, Ning-Yu Wang
https://doi.org/10.1016/j.bioorg.2020.104344
- E3 ligase ligand chemistries: from building blocks to protein degraders
Publication Date: 08 Apr 2022
Izidor Sosič, Aleša Bricelj and Christian Steinebach