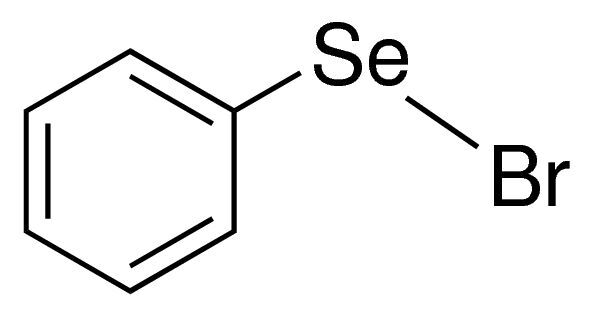
Phenylselenyl bromide
Safety Information
Hazard Statements
Precautionary Statements
Pictograms
Product Description
Phenylselenyl bromide is an organoselenium compound used in organic synthesis. It features a phenyl group bonded to selenium and bromine, making it reactive in various chemical reactions.
Application:
Phenylselenyl bromide is used as a reagent in organic synthesis, particularly for introducing selenium into molecules and facilitating oxidative reactions.
Articles:
- Regiodivergent metal-catalyzed B(4)- and C(1)-selenylation of o-carboranes
Publication Date: 3rd December 2022
Kyungsup Lee, Jordan L. Harper, Tae Hyeon Kim, Hee Chan Noh, Dongwook Kim, Paul Ha-Yeon Cheong and Phil Ho Lee
https://doi.org/10.1039/D2SC05590B
- Electrophile-induced generation of cyclic azomethine imines from steroidal δ-alkenyl hydrazones
Publication Date: Available online 13 January 2009
Erzsébet Mernyák, László Márk, Éva Frank, Gyula Schneider, János Wölfling
https://doi.org/10.1016/j.steroids.2009.01.001
- X=Y–ZH Systems as potential 1,3-dipoles. Part 54: Stereo- and facially-selective formation of bridged bicyclic N-heterocycles via a sequential one-pot electrophile induced oxime→nitrone→cycloaddition sequence. Multiplication of chirality
Publication Date: Available online 13 June 2002
H Ali Dondas, Ronald Grigg, Sylvie Thibault, W Anthony Thomas, Mark Thornton-Pett