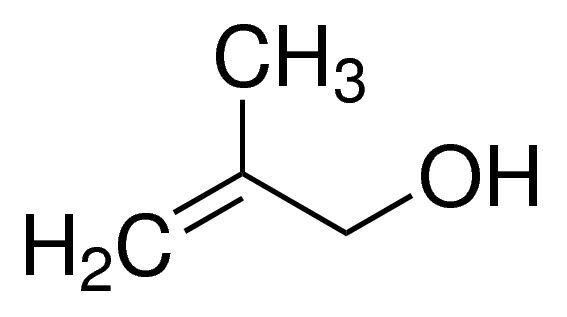
2-Methyl-2-propen-1-ol
Safety Information
Hazard Statements
Precautionary Statements
Pictograms
Product Description
2-Methyl-2-propen-1-ol is a colorless liquid with a characteristic odor, commonly used as a chemical intermediate in the synthesis of various organic compounds. It exhibits reactivity with a range of functional groups, making it valuable in organic chemistry applications.
Application:
2-Methyl-2-propen-1-ol is utilized as a key building block in the production of pharmaceuticals, agrochemicals, and specialty chemicals. It is particularly employed in the synthesis of antioxidants, flavors, fragrances, and polymer additives due to its versatile chemical properties.
Articles:
- Reactions of [RuClH(CO)(PPh3)3] with 2-methyl-2-propen-1-ol. Reversible insertion/β-elimination, and reductive elimination on a 3-hydroxy-2-methylpropyl-C1,O-ruthenium(II) complex
Publication Date: Available online 10 July 2001
Katsuma Hiraki, Akihiro Nonaka, Takahiro Matsunaga, Hiroyuki Kawano
https://doi.org/10.1016/S0022-328X(98)00932-2
- Kinetics of the reactions of Cl atoms with 2-buten-1-ol, 2-methyl-2-propen-1-ol, and 3-methyl-2-buten-1-ol as a function of temperature
Publication Date: 12 June 2008
Diana Rodriguez, Ana Rodriguez, Amparo Soto, Alfonso Aranda, Yolanda Diaz-de-Mera & Alberto Notario
https://doi.org/10.1007/s10874-008-9101-6
- Rate Coefficients for the Reaction of OH with a Series of Unsaturated Alcohols between 263 and 371 K
Publication Date: April 24, 2008
Pablo M. Cometto, Pablo R. Dalmasso, Raúl A. Taccone, Silvia I. Lane, Fátima Oussar, Véronique Daële, Abdelwahid Mellouki and Georges Le Bras