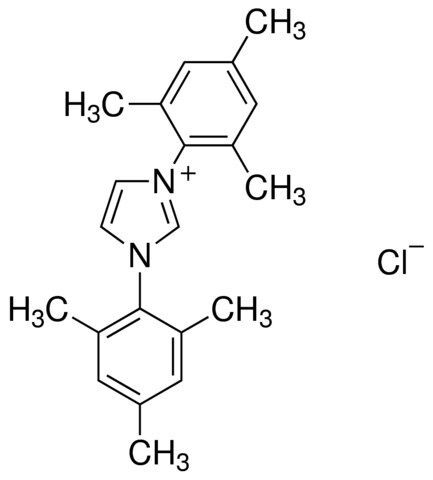
1,3-Bis(2,4,6-trimethylphenyl)imidazolium chloride
Safety Information
Hazard Statements
Precautionary Statements
Pictograms
Product Description
1,3-Bis(2,4,6-trimethylphenyl)imidazolium chloride is a highly effective organocatalyst utilized in chemical reactions that demand high efficiency and specificity. Its unique molecular design, featuring electron-donating trimethylphenyl groups, enhances its catalytic activity across a broad spectrum of reactions. This compound is particularly adept at promoting reactions involving difficult substrates, leading to increased yields and selectivities. Its robust performance under various reaction conditions makes it a preferred choice for both academic research and industrial applications, especially in the synthesis of complex organic compounds and pharmaceutical intermediates.
Application
In the field of chemistry, 1,3-Bis(2,4,6-trimethylphenyl)imidazolium chloride acts as an organocatalyst, significantly improving the efficiency and selectivity of chemical reactions. Its use extends from laboratory environments to industrial settings, where it aids in the production of pharmaceuticals, agrochemicals, and other specialty chemicals. This compound's ability to catalyze reactions under mild conditions contributes to the development of greener and more sustainable chemical processes, aligning with current trends towards environmentally friendly manufacturing practices.
Articles:
- Reaction of nickelocene with 1,3-dimesitylimidazolium chloride
Publication Date: Available online 8 March 2000
Colin D Abernethy, Alan H, Cowley, Richard A Jones
https://doi.org/10.1016/S0022-328X(99)00557-4
- Synthesis and Characterization of 14-Electron Cyclopentadienyl Chromium(II) Complexes Containing a Heterocyclic Carbene Ligand
Publication Date: January 20, 1999
Mark H. Voges, Christian Rømming and Mats Tilset
https://doi.org/10.1021/om980799b
- Efficient telomerization of 1,3-butadiene with alcohols in the presence of in situ generated palladium(0)carbene complexes
Publication Date: Available online 26 March 2002
Ralf Jackstell, Anja Frisch, Matthias Beller, Dirk Röttger, Michael Malaun, Benno Bildstein