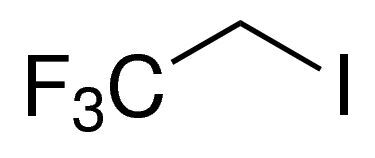
1,1,1-Trifluoro-2-iodoethane
Safety Information
Hazard Statements
Precautionary Statements
Pictograms
Product Description
1,1,1-Trifluoro-2-Iodoethane is a halogenated ethane derivative featuring both fluorine and iodine substitutions. It is used in the synthesis of various organic compounds due to its unique reactivity profile. This compound is particularly valued in the production of specialty chemicals, where its fluoroiodo motif can lead to the creation of molecules with enhanced properties, such as improved solubility or reactivity.
Application:
Applied in the synthesis of specialty chemicals, where its fluoroiodo functionality offers unique opportunities for the development of new materials and intermediates with tailored properties. Its use spans across industries, including pharmaceuticals, agrochemicals, and materials science, highlighting its versatility in chemical synthesis.
Articles:
- Palladium-Catalyzed Trifluoroethylation of Terminal Alkynes with 1,1,1-Trifluoro-2-iodoethane
Publication Date: February 1, 2013
Yi-Si Feng, Chuan-Qi Xie, Wen-Long Qiao and Hua-Jian Xu
-https://doi.org/10.1021/ol400099h
-Nickel-Catalyzed Direct Trifluoroethylation of Aryl Iodides with 1,1,1-Trifluoro-2-Iodoethane via Reductive Coupling
Publication Date: 24 September 2020
Han Li, Jie Sheng, Guang-Xu Liao, Bing-Bing Wu, Hui-Qi Ni, Yan Li, Xi-Sheng Wang
-https://doi.org/10.1002/adsc.202000985
-Visible-Light-Induced Photocatalysis of 1,1,1-Trifluoro-2-iodoethane with Alkylalkenes and Silyl Enol Ethers
Publication Date: 16 September 2015 (online)
Meiwei Huang, Lun Li, Zhi-Gang Zhao, Qing-Yun Chen, Yong Guo